The skin is the largest organ in the human body. Most people don’t realize the critical role skin plays in the survival of multi-cellular organisms. The skin is air tight and provides a mechanical and defensive barrier between the outside environment and the body’s internal structures. It is what holds us together, gives us shape, if you will, and this multifaceted structure is perhaps the less appreciated out of all the body’s organs. The skin contains blood vessels, nerves, sweat glands, specialized glands to ward off pathogens, immune cells, sense receptors, hair, and muscle. And that’s not even the half of it. Within the skin’s three distinctly different layers, its components are specifically arranged to produce a dynamic organ with the capabilities to repair itself. It is for these reasons, and many more, that makes the skin one of the most interesting organs of the body.
Many of the skin’s functions developed out of our need to survive. For example, the malanocyte is a specialized skin cell that helps the body ward off ultraviolet radiation by darkening our skin.
 Another evolutionary adaptation is the goose bump, that prickly raising of the skin that occurs when one is cold or senses danger. What exactly is the goose bump, and why is it important? Let’s take a look at a simplified cross-section of human skin. As mentioned, mammalian skin has three layers. The epidermis, the dermis and the hypodermis. Each hair is contained in a sheath of epidermal cells called a hair follicle. This follicle is associated with sebaceous glands, capillaries, and a microscopic smooth muscle bundle called the arrector pili. This tiny muscle bundle attaches the hair follicle to the underside of the epidermis.
Another evolutionary adaptation is the goose bump, that prickly raising of the skin that occurs when one is cold or senses danger. What exactly is the goose bump, and why is it important? Let’s take a look at a simplified cross-section of human skin. As mentioned, mammalian skin has three layers. The epidermis, the dermis and the hypodermis. Each hair is contained in a sheath of epidermal cells called a hair follicle. This follicle is associated with sebaceous glands, capillaries, and a microscopic smooth muscle bundle called the arrector pili. This tiny muscle bundle attaches the hair follicle to the underside of the epidermis.
Let’s now look at the mechanics of the goose bump formation. Ordinarily, the hair emerges from the skin at an angle. When the arrector pili muscle fibers contract, this action pulls the lower half of the hair follicle toward the epidermis, resulting in the hair standing more erect. The result of this creates a “bumping” of the skin surrounding the shaft of hair above the skin. You now have a goose bump.
So, that’s the mechanics, now let’s take a look at the cause of a goose bump. Goose bumps are the effect of activation of the sympathetic branch of the autonomic nervous system. As mentioned above, the skin is innervated by nerves. Nerve cells respond to stimuli in the body and produce neurotransmitters, small molecules that bind to receptors on a cell surface. The arrector pili muscle cells contain alpha 1 (α 1) adrenergic receptors which bind to the neurotransmitter norepinephrine. The release of norepinephrine from the neuron follows the “fight-or-flight” response. When a person senses danger, surprise, or the body is under certain stress, the neuron receives a message and responds by releasing nor-epi. Norepinephrine then binds to the cell surface, and series of events occur within the cell that causes the arrector pili to contract.
Why do we have goose bumps? The answers are quite simple. Our ancestors had more hair than we did. To withstand the frigid temperatures the arrector pili was stimulated, causing the hairs to stand on end and trapping warmer air closer to the skin. It provided our ancestors with the thermal layer they needed. Erect hair also shows dominance. This is seen today when the hairs of a dog stand on end. Perhaps long ago our ancestors displayed the same sort of aura to their rivals. Imagine 150,000 years ago if you were face to face with your enemy who had hair sticking out like a pekingese dog. My guess would be you would do more than laugh. In a more practical sense, when someone becomes frightened or threatened, the “fight-or-flight” response kicks in, and a host of reactions take place. Heart rate increases, digestive functions decreases, skeletal muscles contract, eyes dilate, blood flow increase, hair stands on end. This reaction helps us fight, or allows us to get out of the way fast. It most likely is a reason for the success of Homo sapiens as a species.
The average adult has about 5 million hair follicles. That’s an awful lot of goose bumps.

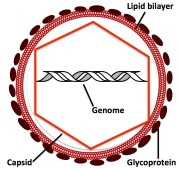 r with many scientists due to the molecular unraveling of evolutionary histories of the species, but also the idea of what constitutes a living organism has been put on trial.
r with many scientists due to the molecular unraveling of evolutionary histories of the species, but also the idea of what constitutes a living organism has been put on trial.


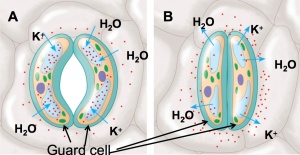 o the atmosphere. Let’s take a look at how this happens. The concept is simple. When water enters the guard cells, the cells swell and become turgid, and because they are attached at their ends, the space between them widens. Why does water enter the guard cells in the first place? Water diffuses from a low to a high concentration of solute. Potassium (K+ ) first enters the guard cell from neighboring cells, and as a result, water follows and the stomata open. This is shown at A on the drawing to the left. When K+ leaves the guard cells, water follows, the guard cells become flaccid and the stomata close. This is illustrated at B.
o the atmosphere. Let’s take a look at how this happens. The concept is simple. When water enters the guard cells, the cells swell and become turgid, and because they are attached at their ends, the space between them widens. Why does water enter the guard cells in the first place? Water diffuses from a low to a high concentration of solute. Potassium (K+ ) first enters the guard cell from neighboring cells, and as a result, water follows and the stomata open. This is shown at A on the drawing to the left. When K+ leaves the guard cells, water follows, the guard cells become flaccid and the stomata close. This is illustrated at B.

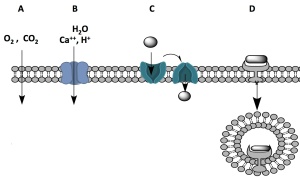 “gate-keep,” allowing only small, hydrophobic molecules such as O2 and CO2 to move through it unaided through the process of passive diffusion. This is illustrated in “A.” In this way, O2 is delivered quickly and efficiently into the cells when needed and its entry doesn’t depend on the presence of a protein, which might ultimately slow down the process because it takes a while for the proteins to make their way to the cell membrane. Despite their size, steroid hormones, such as testosterone and progesterone, can also pass through the cell membrane this way because they are fat-soluable and are not impeded by the membrane’s hydrophobic interior.
“gate-keep,” allowing only small, hydrophobic molecules such as O2 and CO2 to move through it unaided through the process of passive diffusion. This is illustrated in “A.” In this way, O2 is delivered quickly and efficiently into the cells when needed and its entry doesn’t depend on the presence of a protein, which might ultimately slow down the process because it takes a while for the proteins to make their way to the cell membrane. Despite their size, steroid hormones, such as testosterone and progesterone, can also pass through the cell membrane this way because they are fat-soluable and are not impeded by the membrane’s hydrophobic interior.


